Choose your country or language
- Global English
by country or language
- Bosnia and Herzegovina Bosanski
- Bulgaria български
- China 简体中文
- Hong Kong 中文
- Taiwan 繁體中文
- Croatia Hrvatski
- Czech čeština
- Belgium Nederlands
- Netherlands Nederlands
- Greece ελληνικά
- UAE English
- Australia English
- Aserbaidschan English
- Egypt English
- Ethiopia English
- United Kingdom English
- Hong Kong English
- India English
- Lebanon English
- Malaysia English
- Nigeria English
- Philippines English
- Pakistan English
- Thailand English
- United States English
- South Africa English
- Argentina Español
- Chile Español
- Colombia Español
- Spain Español
- Mexico Español
- El Salvador Español
- Iran ایرانی
- Finland Suomi
- Belgium Français
- France Français
- Luxembourg Français
- Morocco Français
- Tunisia Français
- Germany Deutsch
- Hungary Magyar
- Indonesia Bahasa Indonesia
- Italy Italiano
- Japan 日本語
- Korea 한국어
- Poland Polski
- Brazil Português
- Portugal Português
- Belarus Русский
- Russia Русский
- Serbia Srpski
- Slovakia Slovenský
- Slovenia Slovenski
- Sweden Svensk
- Turkey Türkçe
- Ukraine Українська
- Vietnam Tiếng Việt

DQS Complaint and appeal process
As a customer-oriented company, it is our goal to continuously provide the best possible service. It is therefore important for us to know how we are perceived by our customers and prospects. We therefore look forward to your feedback, even if your expectations were not met. We will contact you immediately and try to find a solution.
A complaint is an expression of dissatisfaction, other than appeal, by any interested party to DQS, relating to certification activities of DQS, its staff, Representatives and Auditors or to its certified DQS customers, where a formal response is explicitly or implicitly expected.
An appeal is defined as a request by a DQS client for reconsideration of a certification decision. Appeals are decided by a separate impartial technical reviewer (preferably the respective program manager) and not involved in the conduct of the audit nor the initial decision. Before decision making, responsible management of the accredited DQS entity shall be consulted. The process shall not affect nonconformity management or certificate decertification timelines.
The submission, investigation and decision on complaints or appeals shall not result in any discriminatory actions against the complainant or appellant.
To file any complaints or appeals with the notified body DQS Medizinprodukte GmbH, kindly email your concerns to beschwerde@dqs-med.de. You will find a description below at the bottom of the page. We will contact you immediately and make every effort to find a solution. All other complaints or appeals for any other entity shall be entered below.
However, if you wish to report a violation by DQS auditors or employees of our DQS Policies, Values, Code of Ethics or any law, please go to the https://www.dqsglobal.com/intl/contact/violation
DQS Holding complaint process
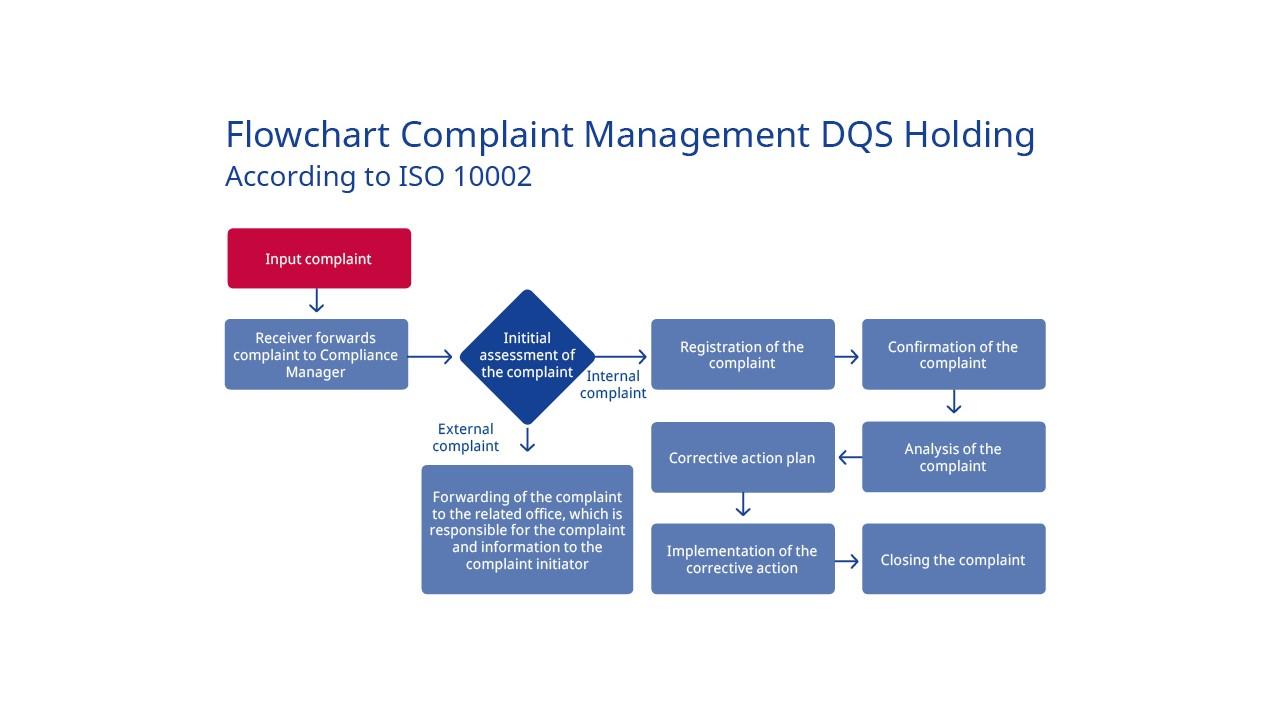
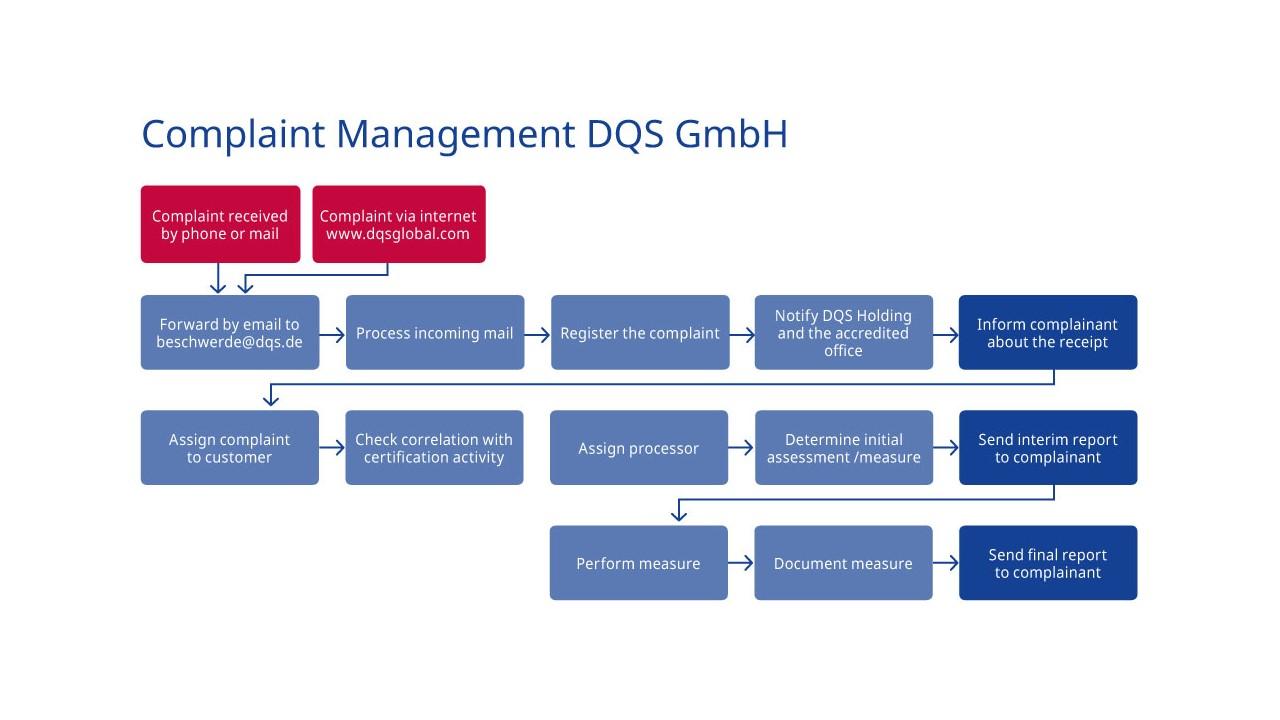
DQS GmbH complaint process
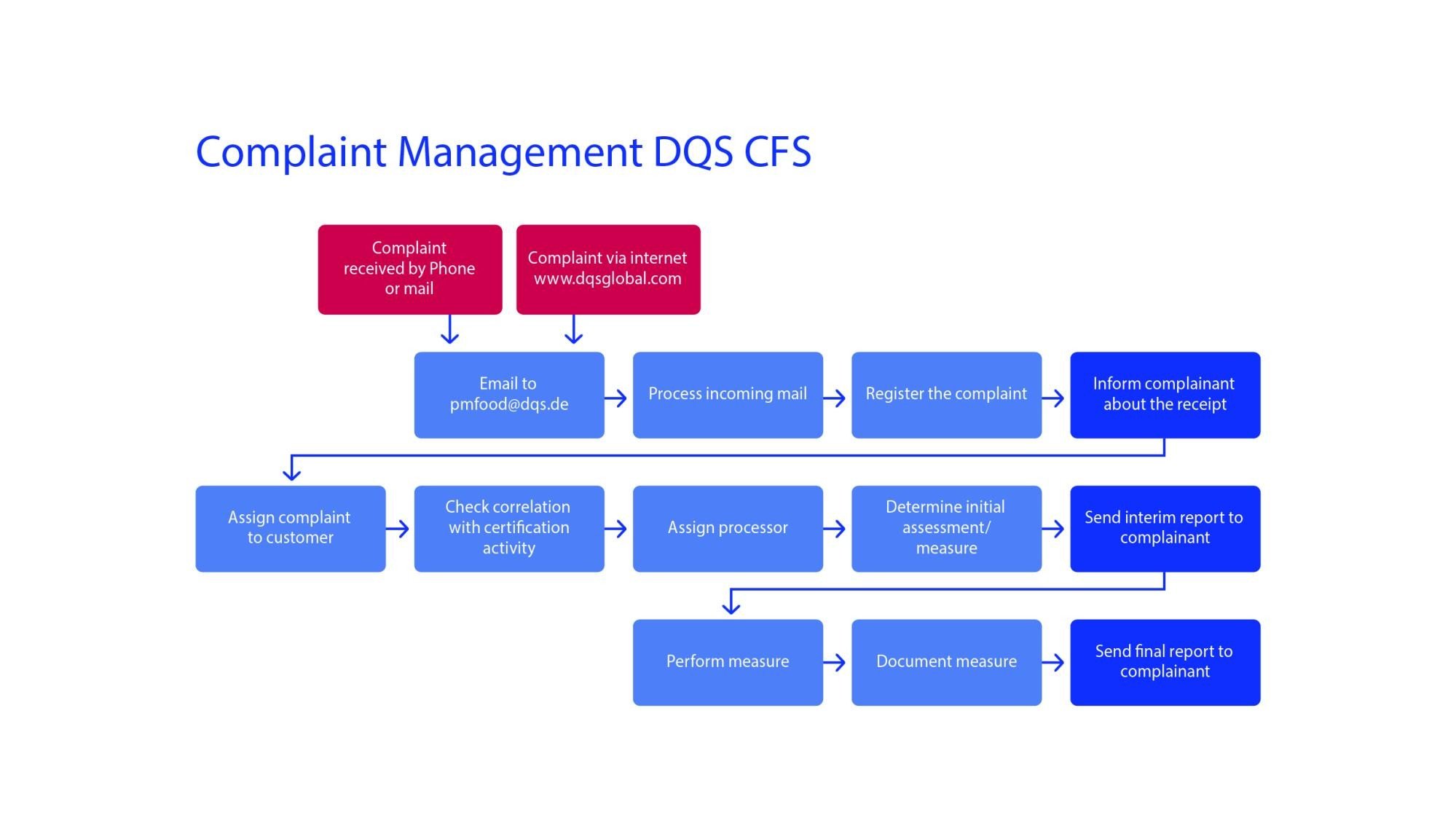
DQS CFS GmbH complaint process
Complaints / Appeals - DQS Medizinprodukte GmbH
We as a certification body / notified body are open to complaints (ISO/IEC 17021-1:2015 Chapter 4.7) and handle them responsibly (ISO/IEC 17021-1:2015 Chapter 9.7.1 and 9.8.1). The filing, investigation and adjudication of a complaint or appeal against a certification decision does not prejudice the complainant (ISO/IEC 17021-1:2015 Chapter 9.7.3 and Chapter 9.8.2). A complaint or an appeal can be submitted in German or English language, is electronically recorded, clearly marked and the course of processing is recorded.
Procedural steps for processing (The processing of a case applies equally to complaints and appeals):
1. Submit (via email to beschwerde@dqs-med.de, or contact your account manager).
2. Your case will be registered internally and identified with a unique case number, you will receive a confirmation of receipt with the case number, which you should always include in the subject line in further communication.
3. Independence and neutrality are ensured by not including employees involved in the process in the decision on the process.
4. The case and the attached evidence will be analyzed, and the parties will provide their comments and justifications in writing. If necessary, you will be asked to provide further information for the decision-making process.
5. After analysis, a decision is made on further action, including possible corrections and corrective measures, as well as possible measures for internal improvement.
6. Finally, you will receive a message and the result of your process.
If you have further detailed questions about the process, please ask them during the processing of your case. We will be happy to answer them.