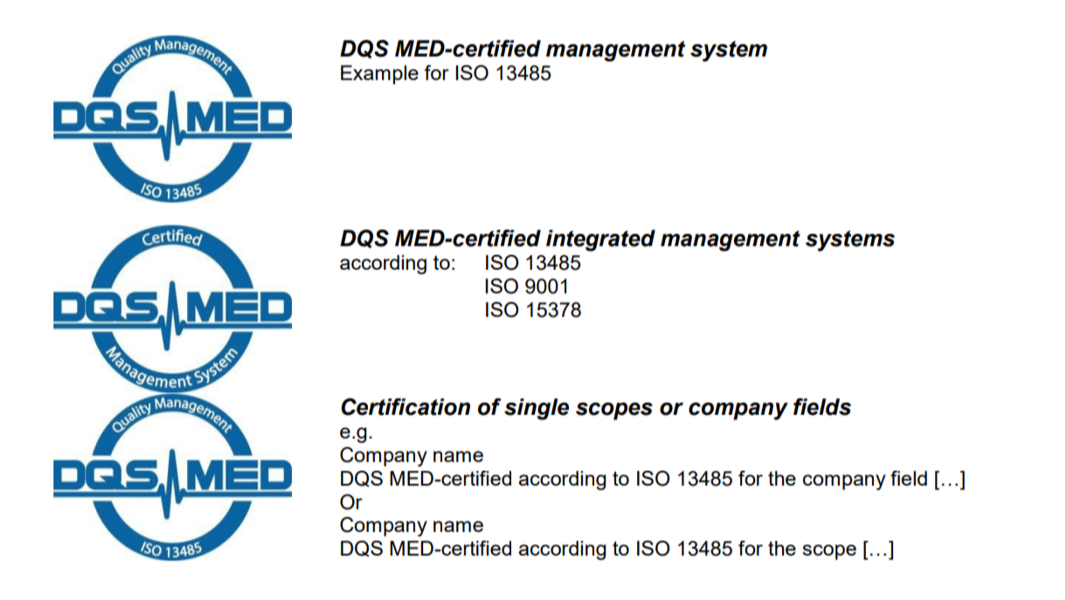
Below you will find various certification logos and symbols sorted according to the respective regulations for use after a successful certification.
Good to know
The ZIP files available for download contain the following formats:
- EPS: for print media
- JPG: for use on websites, presentations, etc.
Please note that you need to unzip these files before you can work with the image files.
Set your mark! DQS MED and IQNet certificates as well as the copyrighted certification symbols are the outer markings of a successful certification – and enjoy an excellent reputation anywhere in the world.
The symbols may be used in a variety of ways: you can put them on your letterhead stationery, on your brochures, or on your vehicles. You can also use them in all your advertisement, at trade fairs or on the Internet.
But please note:
- Use of the certification symbols requires valid certificates or other declarations of conformity issued by DQS MED
- If your certificate does not apply to the whole of your organization, you are requested to indicate the restricted scope for which it is valid
- The symbols should always be used in connection with the name of your company
- When using a neutral certification symbol, the underlying standards or specifications need to be specified in the immediate vicinity of the symbol
- The symbols may not be changed in any way – however, you may choose which color to use
- The IQNet symbol may be used along with the DQS MED certification symbol, but not by itself
- We recommend appending the certificate registration number to the symbol
- The symbols may not be used for the labeling of products
- The DQS MED and IQNet logos (symbols without the rings) are not certificate symbols. They are protected as proprietary marks virtually world-wide, and DQS MED and IQNet reserve them for their own use.
- The DQS MED’s policy for the use of certification symbols is to be followed strictly.
Please see Annex for some design examples.
For organizations with their own website, we recommend placing the information of your successful certification there as well. You may also want to include a link to the customer data base on the DQS Medizinprodukte GmbH’s website – www.dqs-med.de, in order to demonstrate the validity of your certification and for your own legal certainty.
Certificate symbols are available in digital format on the DQS Medizinprodukte GmbH’s website, “good to know” section.
Annex “Rules for the use of certification symbols“
Samples
Symbols on products or packaging
These symbols may not be used for the labeling of products.
The impression of a product certification must be avoided at all costs.
The following chart is designed to assist in how to use symbols to show that the product was manufactured
using a certified management system.
Use of DQS MED and IQNet certificate symbol
Without further explanation:
On the product *1 - Not allowed
On large boxes and similar containers used for the transport of products *2 - Not allowed
In brochures etc. for advertising purposes - Allowed
With further explanation*3:
On the product *1 - Not allowed
On large boxes and similar containers used for the transport of products *2 - Allowed
In brochures etc. for advertising purposes - Allowed
*1) Either on the product itself or on its individual packaging, container etc. In case of inspection/analysis services, this may also be the report of inspection/analysis.
*2) This may be oversized packaging of cardboard or such, which will not reach the end user.
*3) This may be a statement that, for example, the product was manufactured using a DQS MED-certified management system to ISO 13485. Instead of “manufactured“, it may also read “packaged“, or “supplied“, as applicable. For example: “Packaged by an organization with a DQS MED- certified management system”.